Abstract
INTRODUCTION: Our group has demonstrated that MASS-FIX is a quick, inexpensive, and accurate means to diagnose and monitor the serum and urine of patients with plasma cell disorders. Screening can be done with a MALDI-TOF MS and samples reflexed to microflow liquid chromatography coupled with electrospray ionization (ESI) and Q-TOF MS (microLC-ESI-Q-TOF MS). Because this technique provides a mass/charge (m/z) for a given patient's monoclonal protein, this method can provide greater sensitivity and specificity to monitor for complete response (CR), especially in patients receiving therapeutic monoclonal antibodies. Our goal was to assess the performance of miRAMM in patients with AL who have been classified as complete response using conventional means.
METHODS: We identified 77 patients with AL who had both documented CR by immunofixation (Seibia) of the serum (SIFE), urine IFE (UIFE), and serum free light chain (FLC; The Binding Site) and paired serum samples to test by MALDI-TOF and ESI-TOF. No urine samples were available to test. Paired serum samples from baseline and approximately one year post-therapy were immunoaffinity purified using nanobodies targeting kappa, lambda, alpha, gamma and mu as previously described. For the MALDI-TOF (Bruker Microflex, LT), a range of 9,000 to 32,000 m/z was acquired. The m/z distribution was then visually inspected for the presence of a peak that was distinct from the polyclonal background in both the M+1 and M+2 light chain mass ranges. For the ESI-TOF, spectra were also collected on an TripleTOF 5600 quadrupole time-of-flight mass spectrometer (ABSciex, Vaughan ON, CA) in ESI positive mode with a Turbo V dual ion source with an automated calibrant delivery system. TOF MS scans were acquired from m/z 600−2500 with an acquisition time of 100 ms.
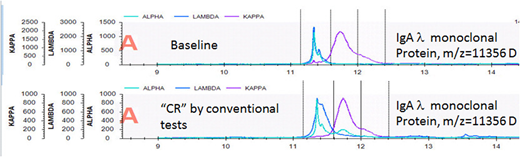
RESULTS: Median age of the cohort was 58 (range 42, 81). Fifty-eight percent were male. No test was 100% sensitive at baseline with positive results as follows: abnormal FLC ratio, 82%; positive SIFE, 70%; 73% positive UIFE; positive serum MASS-FIX, 71%; and ESI-TOF, 79% . There was light chain isotype agreement in all cases, except for 2 patients for whom the SIFE and the FLCr disagreed. Of the 56 patients with baseline positive MASS-FIX, there was evidence of the original monoclonal protein in 5 patients (see figure for example of spectra illustrating same m/z before and after therapy). Of the 63 patients who had baseline positive ESI-TOF, there was evidence of the original monoclonal protein in 8 patients. Small unrelated monoclonal proteins were seen both by SIFE and by mass spectrometry techniques. With the spectrometry techniques, however, these transient oligoclonal bands could be distinguished from the original monoclonal protein when they shared the same isotype based on the difference in m/z.
DISCUSSION: At baseline, the MALDI was able to identify the baseline monoclonal protein in a comparable number of patients as SIFE, UIFE, and FLC. Approximately 10% of patients thought to be in CR using routine screening tests were found to have persistence of their original clone by MALDI and by ESI-TOF. Small post-therapy unrelated monoclonal proteins were also seen both by IFE and by MALDI, but either the presence of a different isotype or in the case of MALDI, a different m/z, made it clear that the monoclonal protein was not related to the original clone. The sensitivity of the assay will improve significantly when we formalize the introduction of free light chain magnetic beads to capture the serum FLCs. Routine use of MASS-FIX of the urine will also increase performance characteristics.
Dispenzieri:Celgene, Takeda, Prothena, Jannsen, Pfizer, Alnylam, GSK: Research Funding. Gertz:Prothena: Honoraria; celgene: Consultancy; spectrum: Consultancy, Honoraria; Teva: Consultancy; Physicians Education Resource: Consultancy; annexon: Consultancy; Apellis: Consultancy; Alnylam: Honoraria; Medscape: Consultancy; Amgen: Consultancy; janssen: Consultancy; Research to Practice: Consultancy; Abbvie: Consultancy; Ionis: Honoraria. Kumar:Novartis: Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Dingli:Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.; Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.; Millennium Takeda: Research Funding; Millennium Takeda: Research Funding. Kapoor:Takeda: Research Funding; Celgene: Research Funding. Russell:Vyriad: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal